According to Pauli Exclusion Principle, no two electrons in an atom can have the same set of four identical quantum numbers. It means, if any two electrons have same values of n, s, ml (as we just discussed above) then the l value would definitely be different in them. Hence, no two electrons will have same energy.
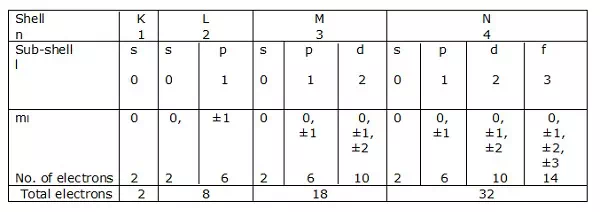
Electronic shells
If n = 1 is a shell, then l = 0 is a sub-shell.
Likewise, n = 2 is a shell, and l = 0, 1 is a sub-shell.
Shells of electrons corresponding to n = 1, 2, 3….. are represented by K, L, M, N respectively. The sub-shells or the orbitals corresponding to l = 0, 1, 2, 3 etc. are denoted by s, p, d, f etc. respectively.
Let us have a look at the electronic configurations of carbon, silicon and germanium (Group IV – A).
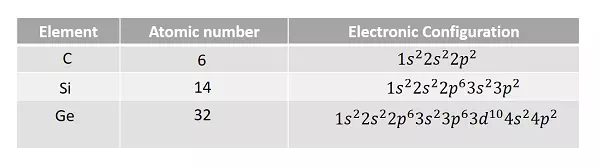
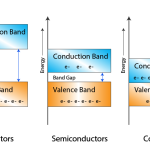
It is observed that the outermost p sub-shell in each case contains only two electrons. But the possible number of electrons is six. Hence, there are four valence electrons in each outer most shell. So, each electron in an atom has specific energy. The atomic arrangement inside the molecules in any type of substance is almost like this. But the spacing between the atoms differ from material to material.
Comments are closed