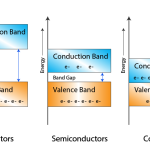
The gap between valence band and conduction band is called as forbidden energy gap. As the name implies, this band is the forbidden one without energy. Hence no electron stays in this band. The valence electrons, while going to the conduction band, pass through this.
The forbidden energy gap if greater, means that the valence band electrons are tightly bound to the nucleus. Now, in order to push the electrons out of the valence band, some external energy is required, which would be equal to the forbidden energy gap.
The following figure shows the valance band, conduction band, and the forbidden gap.
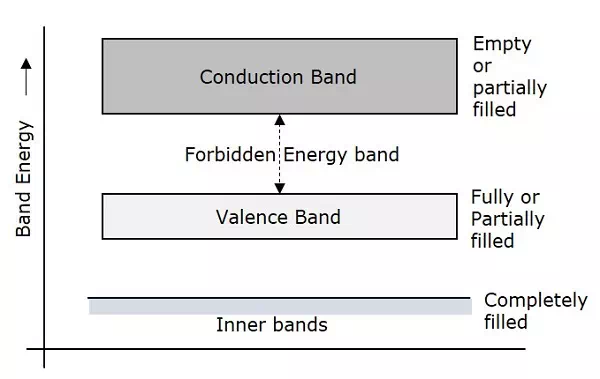
Depending upon the size of the forbidden gap, the Insulators, the Semiconductors and the Conductors are formed.