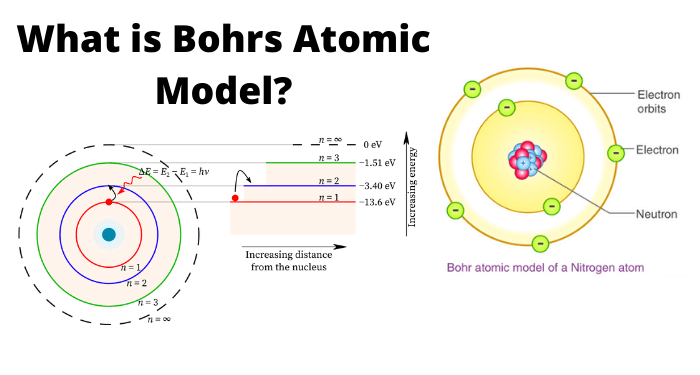
Matter is made up of molecules which consists of atoms. According to Bohr’s theory, “the atom consists of positively charged nucleus and a number of negatively charged electrons which revolve round the nucleus in various orbits”. When an electron is raised from a lower state to a higher state, it is said to be excited. While exciting, if the electron is completely removed from the nucleus, the atom is said to be ionized. So, the process of raising the atom from normal state to this ionized state is called as ionization.
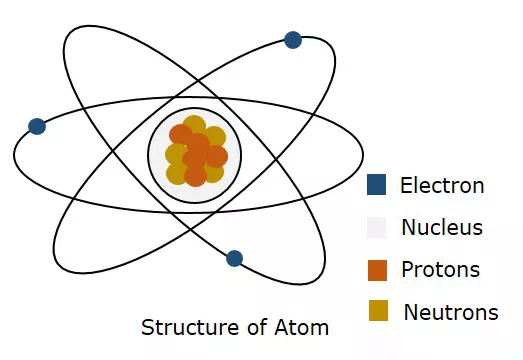
The following figure shows the structure of an atom.
According to Bohr’s model, an electron is said to be moved in a particular Orbit, whereas according to quantum mechanics, an electron is said to be somewhere in free space of the atom, called as Orbital. This theory of quantum mechanics was proven to be right. Hence, a three dimensional boundary where an electron is probable to found is called as Atomic Orbital.