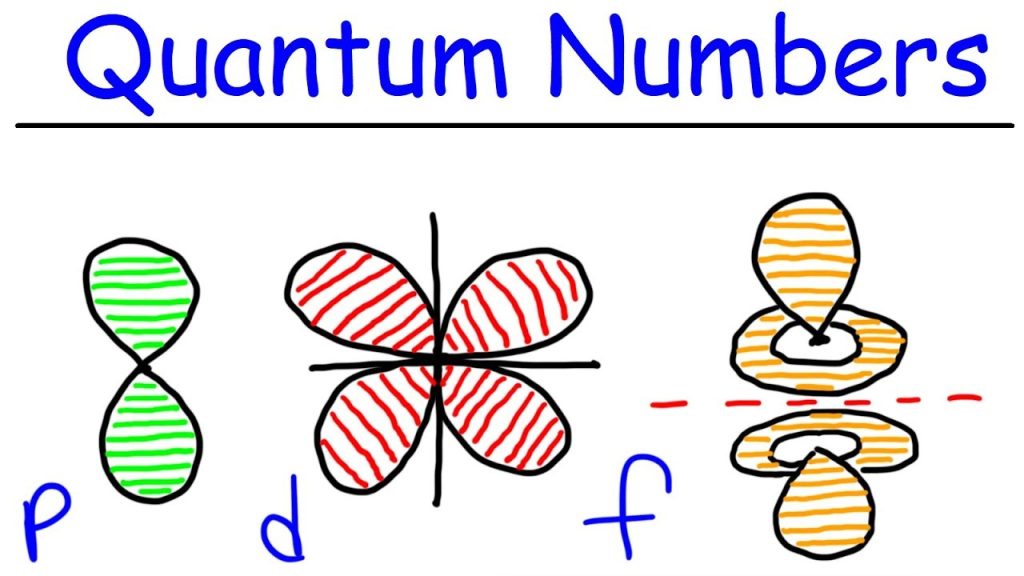
Each orbital, where an electron moves, differs in its energy and shape. The energy levels of orbitals can be represented using discrete set of integrals and half-integrals known as quantum numbers. There are four quantum numbers used to define a wave function.
Principal Quantum number
The first quantum number that describes an electron is the Principal quantum number. Its symbol is n. It specifies the size or order (energy level) of the number. As the value of n increases, the average distance from electron to nucleus also increases, as well, the energy of the electron also increases. The main energy level can be understood as a shell.
Angular Momentum Quantum number
This quantum number has l as its symbol. This l indicates the shape of the orbital. It ranges from 0 to n-1.
l = 0, 1, 2 …n-1
For the first shell, n = 1.
i.e., for n-1, l = 0 is the only possible value of l as n = 1.
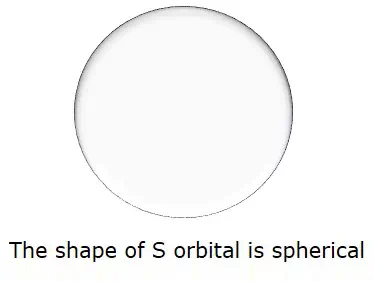
So, when l = 0, it is called as S orbital. The shape of S is spherical. The following figure represents the shape of S.
If n = 2, then l = 0, 1 as these are the two possible values for n = 2.
We know that it is S orbital for l = 0, but if l = 1, it is P orbital.
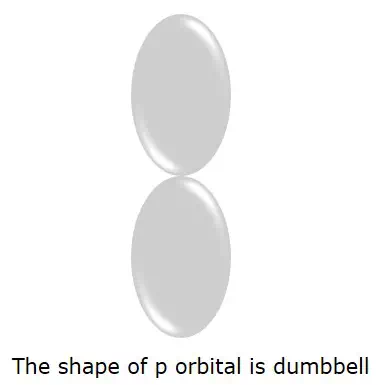
The P orbital where the electrons are more likely to find is in dumbbellshape. It is shown in the following figure.
Magnetic Quantum number
This quantum number is denoted by ml which represents the orientation of an orbital around the nucleus. The values of ml depend on l.
ml=∫(−lto+l)ml=∫(−lto+l)
For l = 0, ml = 0 this represents S orbital.
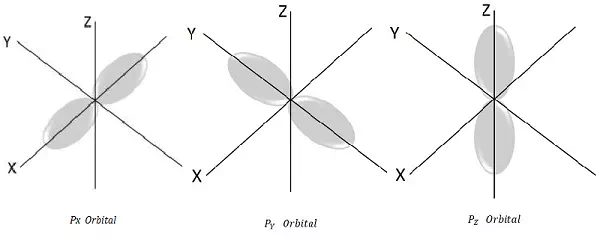
For l = 1, ml = -1, 0, +1 these are the three possible values and this represents P orbital.
Hence we have three P orbitals as shown in the following figure.
Spin Quantum number
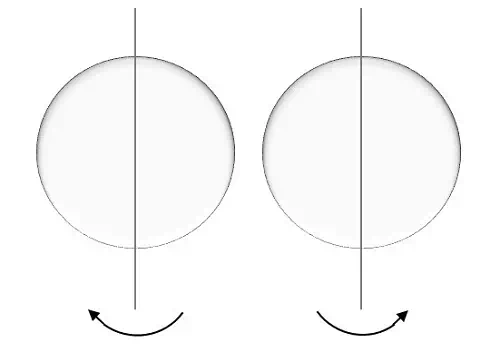
This is represented by ms and the electron here, spins on the axis. The movement of the spinning of electron could be either clockwise or anti-clockwise as shown here under.
The possible values for this spin quantum number will be like,
ms=+12upms=+12up
For a movement called spin up, the result is positive half.
ms=−12downms=−12down
For a movement called spin down, the result is negative half.
These are the four quantum numbers.